Development of best-in-class therapeutics through innovative technology

About Us
At Pharmacin, we are committed to improving patient outcomes through the development of best-in-class therapeutics. From concept through commercialization, we deploy state-of-the-art technology to enable value-added medicines to be realized faster and more cost effectively.
Pharmacin was founded in 2016, and has finished round B of fundraising in 2021. Pharmacin has global, international standards and market strategy mindset.
Headquartered in Shenzhen, China; and a subsidiary in Suzhou, China and another subsidiary, HLK Pharmacin in New Haven, Connecticut, USA.
Shenzhen (China) site is focused on R&D, CMC, clinical development, and capitalization with a high caliber team and state-of-the art R&D capabilities.
Suzhou (China) site is focused on late stage development and commercialization.
New Haven (USA) site is focused on business development and international collaborations, supported by executives with experience at top tier global pharmaceutical companies.



Our Team
Meet our management team

Zeren Wang, PhD
Founder, Chairman and CSO
Dr. Wang received his PhD in Physical Pharmacy from University of Utah under Dr. William Higuchi. As the CSO he is responsible for the overall science and technical management of the company. Prior to this position, Dr. Wang was the Director of Formulation Development, at Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT. In this role, he provided strategic leadership to a team focused on formulation and process development of NCE products. Dr. Wang is specialized in formulation development of poorly water soluble compounds for oral delivery and experienced in the development of oral solid dosage forms (tablets, hard gelatin capsules, soft gelatin capsules), oral liquid dosage forms (solutions and suspensions), controlled release technology, and parenterals (lyophilization). Under his leadership, his team introduced and implemented multiple new formulation technologies including SEDDS (self emulsifying drug delivery system) formulation technologies (soft gel and hard gel encapsulation capabilities), solid dispersion formulation technologies (spray drying and melt extrusion) as well as nano-milling. He has 30 peer-reviewed publications and patents, as well as numerous poster presentations.

Jun Xu, MS
Founder and CEO
Ms. Xu has over 15 years of experience in major international pharmaceutical companies including Novartis and Boehringer Ingelheim in drug discovery and development. She has worked on multiple new drug targets, including target proposals, target validation and assay development for new drug candidates in Immunology and cardiovascular disease areas.
Ms. Xu also worked in drug development focusing on metabolism, anaerobic gut flora systems and drug-drug interactions studies. Overall, she has been involved in the discovery of a dozen drug candidates and development of several new drugs. Ms Xu also served as a core member of the leadership team for the Asian Employee Resource Group at BI and has actively volunteered her time to initiate a number of activities in the Chinese-American community. She founded the Huaxia Chinese School of Connecticut (HXCT). Over the years, Jun served as founder Principal, then the Chair of the Board for HXCT and Board member and Chairperson of Huaxia School headquarter. HXCS has become a central platform of the Chinese-American community.
Ms. Xu’s diverse experience in institutional management has helped her build up an extensive network in the pharmaceutical industry, government, and university communities both in China and the United States.
Ms. Xu has a BS degree from Nanjing University, and a MS degree from SUNY Buffalo.

Deepak Hegde, PhD
Senior Vice President, Technology & Manufacturing
Dr. Deepak Hegde completed his Doctorate in Biopharmaceutics and Pharmacokinetics from University of Mumbai in 1996, and his Masters in Financial Management (M.F.M) from University of Mumbai in 2000.
Prior to Pharmacin, Dr Hegde worked with EOC Pharma as Chief Technology Officer where he was responsible for development and manufacturing of new anti-cancer products for China. Dr Hedge also has served as Director, Global External Development & Supply, AsiaPacific at GSK Shanghai, R & D China where he was responsible for developing drug products for the global portfolio and for introduction of new products to China. Dr Hegde has also held roles at WuXi Apptec as Vice President for Formulation Development, Rhone Poulenc, Sandoz (A Novartis Group Company) and USV Ltd, a premier Indian Generic Company. He is a life member of the Indian Pharmaceutical Association, and has two US patents, five PCT and US patent applications and several National and International publications to his credit.

Lian Wang, PhD
Vice President, Translational
Sciences
Dr. Wang is Vice President of Translational Sciences, responsible for evaluation and managing our pipelines to make highly differentiated and globally competitive medicines.
Prior to joining Pharmacin, Dr. Wang participated in creation of Vivoz Biolabs and served as the CEO of the company. She also worked in R&D centers of Boehringer Ingelheim in the US and Abbott (currently AbbVie) in China to lead/co-lead multi-disciplinary teams on multiple projects. In addition, she was responsible for new target proposals, new target evaluations for in-licensing and external collaborations. Dr. Wang has extensive experience in early drug discovery processes, from target validation, assay development, high-throughput screen, activity evaluation, to lead optimization, etc.
Dr. Wang earned a bachelor’s degree in biochemistry from Beijing University and a doctorate in molecular from University of Pennsylvania, and completed her postdoctoral fellowship training at Harvard University Medical School.
Dr. Wang authored or co-authored in numerous scientific publications in many prestige journals, including Cell.

Shun Chen, PhD
Senior Director, Research &
Development
Dr. Chen is Senior Director of R&D, leading a multi-functional team of scientists ranging from R&D Operations to Physical Pharmaceutics who are responsible for project management, quality assurance, etc, and a Physical Pharmaceuticals team responsible for project evaluations, feasibility assessments, and early-stage drug product development. The team is exploring novel formulation technologies based on the scientific principles of physical pharmacy, including proprietary spray drying technology, peptide oral delivery, and siRNA delivery technologies.
Prior to joining Pharmacin, Dr. Chen was the head of R&D in Humanwell Pharmaceutical Co. Ltd., responsible for new project evaluation and drug product development, and successfully transformed several development products to marketed products.
Dr. Chen earned a doctorate in pharmaceutics from Jiaotong University
Dr. Chen authored or co-authored in a number of scientific publications and is an inventor on multiple patents

Stanley Choy, MBA
Chief Operating Officer
Mr. Choy is an accomplished executive and entrepreneur with over 25 years of cross-functional experience in the healthcare, biotechnology and professional services industries. He is a co-founder of SHY Therapeutics and provides financial and advisory services to early stage, start-up ventures. Mr. Choy’s areas of expertise include financial and strategic planning, operations, business development, and regulatory and government affairs.
Earlier in his career, Mr. Choy served as the Vice President, Finance and Administration at Kolltan Pharmaceuticals, a Yale affiliated spin-out. Prior to joining Kolltan, Mr. Choy was the Chief Financial Officer for a leading New York/Boston based law firm. During his tenure at Pfizer, Mr. Choy held several senior finance positions including finance director of business planning for the Consumer Health Care Division, finance director of the Asia & Latin America Region for the Animal Health Care Division, and finance director of the U.S. and Asia Region for the Corporate Finance and Treasurer’s Division. He was also instrumental in the integration of the Warner Lambert and Pharmacia acquisitions. Mr. Choy started his career with Arthur Andersen and Company.
Mr. Choy serves as a director and co-chair of BioCT, the bioscience industry association for the state of Connecticut.
Mr. Choy is a CPA and has an MBA in finance from Syracuse University and holds an accounting degree. He is certified in New York State and a member of the American Institute of CPAs.

Peter Farina, PhD
Scientific Advisory Board
Chairman
Dr. Farina has extensive experience in the pharmaceutical industry, including a 28 year career at Boehringer Ingelheim in Ridgefield, CT, developing drugs to modulate inflammatory and immunological processes and also engaging in HIV virology research which led to the discovery and successful registration of one of the first non-nucleoside reverse transcriptase inhibitors Viramune® (nevirapine). As Senior Vice President of Research, he was responsible for pre-clinical drug development in North America in the therapeutic areas of immunology/inflammation, virology and cardio-metabolic diseases. Dr. Farina and his interdisciplinary team worked on the development and successful registration of Aptivus®, an HIV protease inhibitor, Viramune XR® for HIV, Atrovent HFA® for COPD/emphysema and Jardiance® for diabetes. Earlier in his career, Dr. Farina served as Vice President of Research.
Dr. Farina is currently an Executive in Residence at Canaan Partners, a venture capital firm located in Westport, CT. He is also the managing partner of Salient Science & Technology, LLC which advises several US and Asian biotech firms on strategic and technical matters in pharmaceutical R&D.
Dr. Farina currently serves as a director and co-chair of BioCT; the Advisory Board of the University of Connecticut School of Pharmacy (Emeritus); member of the NIH Blueprint Neurotherapeutics Network (BPN) Executive Oversight Committee; State of Connecticut Bioscience Innovation Fund Advisory Board, and was a Founder and CEO of Developing World Cures, a nonprofit company focused on neglected diseases.
Dr. Farina has a Ph.D. in organic chemistry from SUNY Buffalo and did postdoctoral work in bioorganic chemistry at Pennsylvania State University. He is the recipient of a honorary doctorate from the University of Connecticut.

What We Do
Focus on global markets in therapeutic areas of unmet needs and develop value-added therapeutics that offer potential to improve patient outcomes and access
Re-formulation for New Drug Products
- With proprietary formulation technologies, Pharmacin develops reformulated drug products that offer potential to improve patient outcomes, including enhanced bioavailability, safety, tolerability, patient compliance and accessversus the marketed drug products.
- In the US market, the re-formulated drug products are eligible for development through the 505b2 pathway, offering faster time to market and reduced development costs.
Re-purposing for New Drug Development
- With international SAB and BD teams, Pharmacin has capabilities to develop repurposed drug products that meet unmet medical needs.
Our Technology
The Pharmacin technology advantage


State-of-the art and patented technology offers the opportunity to develop new therapeutics based on the unmet medical needs of novel or existing compounds:
-
New formulations
Application of the technology can improve the in vivo exposure of poorly water-soluble compounds to improve patient outcomes, including enhancement of efficacy,safety, tolerability; Additionally, the technology can be applied to develop new formulations that offer potential to improve patient adherence and compliance and market access
Pharmacin can deploy these technologies: SpraySol™ (Innovative and proprietary spray drying), melt extrusion, jet milling, and nano milling, with a focus on amorphous solid dispersions.
-
New drug delivery systems
The technology can be applied to Develop new drug delivery routes of administration such as oral, parenteral, buccal, sublingual, intra-nasal, topical, or transdermal offering potential to optimize efficacy and safety, and improve patient outcomes
Pharmacin is actively exploring new technologies, such as oral delivery of peptides and delivery of siRNA by various routes.
Preformulation Platform
The purpose of preformulation is to explore whether an active pharmaceutical ingredient (API) is chemically and physically stable, and bioavailable in its intended delivery route. More broadly, preformulation also involves developability assessment of a new molecule for CMC related issues, as well as the solid form selection of an API.
Preformulation activities involve determining the physicochemical properties of an API, including the pH solubility and stability of its solid form, as well as assessing stability of the API under temperature, moisture, photo and peroxide stressed conditions. For new chemical entities or solid forms of an API, additional developability assessment as well as solid form (including salt form) selection is performed. In addition, the feasibility assessment of non-conventional formulation technologies is also explored for the intended delivery route.
Pharmacin has experience in performing studies to meet the formulation challenges of new chemical entities as well as applications of state-of-the- art pharmaceutical technologies for new drug product development.
Conventional Formulation Platform
Conventional formulation and processing technologies are the most commonly utilized in the pharmaceutical industry. However, the application can be complex due to the challenging physicochemical and biological properties of new chemical entities.
Pharmacin’s state-of-the-art equipment utilizes advanced technologies to resolve complicated CMC issues for the development of new drug products. Through the application of QbD principles, we can develop conventional oral solid dosage forms such as tablets, hard and soft capsules, and oral liquid dosage forms such as solutions and suspensions, as well as parenteral formulations.






Novel Formulation Platform
To resolve challenging physicochemical and biological properties of new chemical entities, non-conventional formulation technologies are often applied. As a company experienced in pharmaceutical dosage form development, we focus on novel formulations of problematic drugs from early stage to late stage development balancing risk assessment, milestone targets and investment.
We are able to develop the following non-conventional formulations to overcome the issues related to physicochemical and biological properties of an active pharmaceutical ingredient.
Amorphous Solid Dispersion (ASD)
Technology
Pharmacin’s SpraySol™ is an innovative and
proprietary spray-drying, amorphous solid
dispersion technology. It can enhance in vivo
absorption of poorly soluble compounds that
have high melting temperatures and high LogP
values (highly hydrophobic). SpraySol™ can
generate amorphous solid dispersions that are
physically stable and create a favorable
microenvironment for dissolution and maintaining
supersaturation, thereby enhancing in vivo
absorption. SpraySol™ technology provides
greater improvement of in vivo absorption as
compared to conventional amorphous solid
dispersion technologies for compounds ranging
from small molecules to molecular
glues/bifunctional conjugates. In addition,
the manufacturing process is scalable.
Self-Emulsifying Drug Delivery System
(SEDDS)
For active pharmaceutical ingredients that
are poorly water soluble with high oil-water
partition-coefficient (LogP), SEDDS
formulation technology may be beneficial to
enhance the bioavailability of these APIs.
Selection of appropriate lipid excipients
that can solubilize the API and emulsify
during dissolution is the key toward
development of a SEDDS formulation with
significantly improved bioavailability.
Pharmacin has a proven track record of developing SEDDS formulations with significantly enhanced bioavailability.
Controlled Release Dosage
Controlled release technologies have been
successfully applied to slow down or extend
the release of API from its dosage form,
reducing Cmax and maintain a more stable
plasma profile in vivo. This could help to
decrease toxicity and related side effects
due to high Cmax and reduce dosing frequency
due to short elimination half-life.
Pharmacin has experience developing extended or modified release dosage forms, based on the needs of achieving relevant plasma profiles.
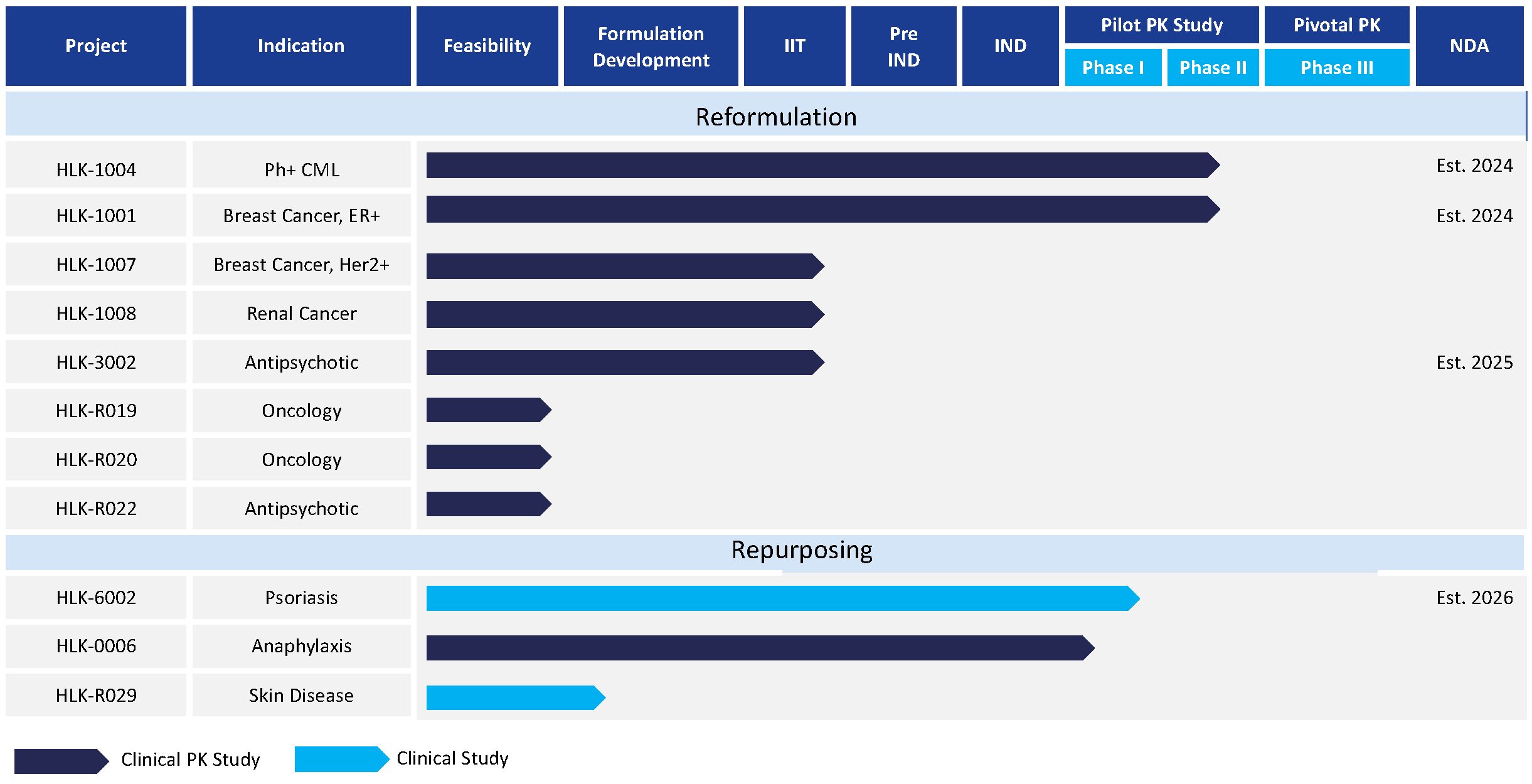
Our Pipeline & Status
Our R&D pipeline
By applying our proprietary formulation platform, Pharmacin has established a pipeline of drug products (with IP protection) for unmet medical needs in several therapeutic areas. The company is actively repurposing and reformulating existing drug products in multi-billion dollar oncology markets following the 505(b)(2) regulatory pathway. The Team has a proven track record in CMC and clinical development, with multiple INDs filed and cleared by FDA. Our lead 505(b)(2) formulation is under development in the US, having recently received IND clearance, and is on track for NDA filing Q4/2024 or Q1/2025.
Our Business
Innovations that transform ideas into differentiated late development stage medicines
• Develop best-in-class 505(b)(2) therapeutics in areas of unmet needs with robust IP for the international market, including China and greater Asia, and the US
• Out-license 505(b)(2) products from our therapeutic portfolio covering cardiovascular, oncology, immunology and allergy
• Lead 505(b)(2) formulation has advanced to clinical development stage in multi-billion dollar hematology/oncology market; and is on track for NDA filing late 2024 or early 2025; IP protection projected to 2041
• Co-develop or in-license candidates for CMC product development for international or Asian markets

* = Required
Contact Us
Learn more about Pharmacin
We pursue drug development challenges with an innovative mindset. Would you like to collaborate with us to advance our patient-focused mission? Contact us today.
470 James Street
Suite 007
New Haven, CT 06513 USA
info@hlkpharma.com
203-267-3309
10 Gaoxin Central 1st Avenue
Bioincubator, Suite 1-303
Nanshan District, Shenzhen 518057
Guangdong Province, P.R. China
info@hlkpharma.com
+86 755-86007001